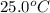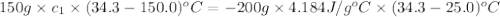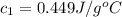Chemistry, 13.11.2019 03:31 champton79
Nunknown metal is either aluminum, iron or lead. if 150. g of this metal at 150.0 °c was placed in a calorimeter that contains 200. g of water at 25.0 °c and the final temperature was found to be 34.3 °c after thermal equilibrium was achieved, assuming heat was only transferred between water and metal, what is the identity of this metal? some specific heat values of metals and water given below may be useful. specific heats, j/(g•°c): fe (0.449) pb (0.128) al (0.903) h2o (4.184)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2
You know the right answer?
Nunknown metal is either aluminum, iron or lead. if 150. g of this metal at 150.0 °c was placed in a...
Questions


Mathematics, 08.12.2020 22:30


Mathematics, 08.12.2020 22:30

Geography, 08.12.2020 22:40


Mathematics, 08.12.2020 22:40

Geography, 08.12.2020 22:40

Mathematics, 08.12.2020 22:40



Biology, 08.12.2020 22:40

History, 08.12.2020 22:40

Business, 08.12.2020 22:40

Arts, 08.12.2020 22:40

Chemistry, 08.12.2020 22:40



Computers and Technology, 08.12.2020 22:40

Mathematics, 08.12.2020 22:40

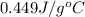 ).
).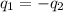
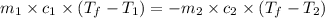
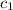 = specific heat of unknown metal = ?
= specific heat of unknown metal = ?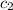 = specific heat of water =
= specific heat of water = 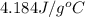
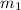 = mass of unknown metal = 150 g
= mass of unknown metal = 150 g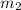 = mass of water = 200 g
= mass of water = 200 g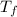 = final temperature of water =
= final temperature of water = 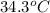
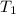 = initial temperature of unknown metal =
= initial temperature of unknown metal = 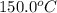
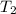 = initial temperature of water =
= initial temperature of water =