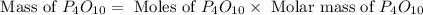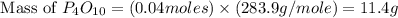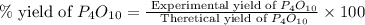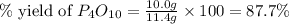Chemistry, 13.11.2019 02:31 gonzalezant8428
The phophorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by nurning phosphorus in oxygen.
(a)what is the limiting reactant when 0.200 mol of p4 and and 0.200 mol of o2 react according to p4 + 5o2 ? p4o10
(b)calculate the percent yield if 10.0 g of p4o10 is isolated from the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1


Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
The phophorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by nurning...
Questions


English, 09.10.2019 22:30

History, 09.10.2019 22:30

Biology, 09.10.2019 22:30




Mathematics, 09.10.2019 22:30

Business, 09.10.2019 22:30

Mathematics, 09.10.2019 22:30



History, 09.10.2019 22:30

Mathematics, 09.10.2019 22:30

Mathematics, 09.10.2019 22:30



Mathematics, 09.10.2019 22:30

Mathematics, 09.10.2019 22:30

Social Studies, 09.10.2019 22:30

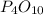 is, 87.7 %
is, 87.7 %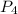 = 0.200 mole
= 0.200 mole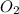 = 0.200 mole
= 0.200 mole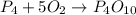
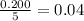 moles of
moles of