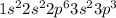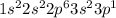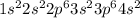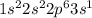Chemistry, 13.11.2019 01:31 serenityarts123
Select the correct statement below:
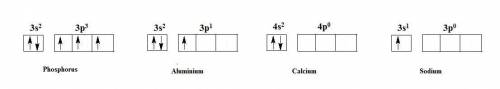
(a) phosphorous contains 10 core electrons and 5 valence electrons. its orbital diagram contains one half-filled 3p orbital and two filled 3p orbitals.
(b) aluminum contains 10 core electrons and 3 valence electrons. its orbital diagram contains three half-filled 3p orbitals.
(c) calcium contains 18 core electrons and 2 valence electrons. its orbital diagram contains two half-filled 4s orbitals and no filled 4p orbitals.
(d) sodium contains 10 core electrons and 1 valence electron. its orbital diagram contains one half-filled 3s orbital and three empty 3p orbitals.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Select the correct statement below:
(a) phosphorous contains 10 core electrons and 5 v...
(a) phosphorous contains 10 core electrons and 5 v...
Questions

Health, 16.12.2019 12:31




Mathematics, 16.12.2019 12:31

Arts, 16.12.2019 12:31

Mathematics, 16.12.2019 12:31

History, 16.12.2019 12:31

Chemistry, 16.12.2019 12:31


Mathematics, 16.12.2019 12:31





Social Studies, 16.12.2019 12:31




Social Studies, 16.12.2019 12:31