Chemistry, 13.11.2019 00:31 ridzrana02
For a particular isomer of c8h18,c8h18, the combustion reaction produces 5099.5 kj 5099.5 kj of heat per mole of c8h18(g)c8h18(g) consumed, under standard conditions. c8h18(g)+252o2(g)⟶8co2(g)+9h2o(g)δh ∘rxn=−5099.5 kj/mol c8h18(g)+252o2(g)⟶8co2(g)+9h2o(g)δh rxn°=−5099.5 kj/mol what is the standard enthalpy of formation of this isomer of c8h18(g)

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
For a particular isomer of c8h18,c8h18, the combustion reaction produces 5099.5 kj 5099.5 kj of heat...
Questions



Mathematics, 16.10.2019 21:30

Mathematics, 16.10.2019 21:30



Biology, 16.10.2019 21:30

Mathematics, 16.10.2019 21:30





English, 16.10.2019 21:30







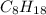 is -222 kJ/mol
is -222 kJ/mol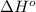
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0371/3800/45485.png)

![\Delta H^o_{rxn}=[(n_{(CO_2)}\times \Delta H^o_f_{(CO_2)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})]-[(n_{(C_8H_{18})}\times \Delta H^o_f_{(C_8H_{18})})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0371/3800/70c63.png)
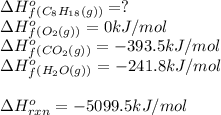
![-5099.5=[(8\times -393.5)+(9\times -241.5)]-[(1\times \Delta H^o_f_{(C_8H_{18})}))+(\frac{25}{2}\times 0)]](/tpl/images/0371/3800/1a9fc.png)