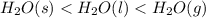Chemistry, 12.11.2019 21:31 msdmdsm1186
Place the following in order of increasing standard molar entropy h2o(l) h2o(g) h2o(s) a)h2o(s)< h2o(l)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
Place the following in order of increasing standard molar entropy h2o(l) h2o(g) h2o(s) a)h2o(s)<...
Questions

Mathematics, 09.08.2021 21:30


Mathematics, 09.08.2021 21:30

Mathematics, 09.08.2021 21:30


Mathematics, 09.08.2021 21:30

Mathematics, 09.08.2021 21:30



Biology, 09.08.2021 21:30

Chemistry, 09.08.2021 21:30

Chemistry, 09.08.2021 21:30

Mathematics, 09.08.2021 21:30

Chemistry, 09.08.2021 21:30

Computers and Technology, 09.08.2021 21:30

Biology, 09.08.2021 21:30

Social Studies, 09.08.2021 21:30

Mathematics, 09.08.2021 21:40