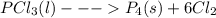Chemistry, 12.11.2019 06:31 gabrielolivas59
4pcl₃(l)  p₄(s) + 6cl₂(g); δh= 304.0 kj
p₄(s) + 6cl₂(g); δh= 304.0 kj
based on the reaction shown, which statement is true?
a) when 1 mol pcl₃(l) reacts, 304.0 kj are released. b) when 1 mol p₄(s) is produced, 304.0 kj are consumed. c) when 548.56 g pcl₃(l) react, 304.0 kj are released. d) when 137.14 g pcl₃(l) react, 304.0 kj are consumed. e) when 123.88 g p₄(s) are produced, 304.0 kj are released.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2

Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
You know the right answer?
4pcl₃(l) [tex]\longrightarrow[/tex] p₄(s) + 6cl₂(g); δh= 304.0 kj
based on the reaction shown...
based on the reaction shown...
Questions


Business, 23.09.2021 23:00



Mathematics, 23.09.2021 23:00



Physics, 23.09.2021 23:00





Mathematics, 23.09.2021 23:00


Geography, 23.09.2021 23:00

Mathematics, 23.09.2021 23:00




Spanish, 23.09.2021 23:10