
Chemistry, 12.11.2019 06:31 freedygotmoney
When an ion‑selective electrode for x+ was immersed in 0.0482 m xcl, the measured potential was 0.0460 v . what is the concentration of x+ when the potential is 0.0610 v ? assume that the electrode follows the nernst equation, the temperature is at 25 °c, and that the activity coefficient of x+ is 1.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1

Chemistry, 23.06.2019 15:40
The poh of a solution is 6.0. which statement is correct? use poh=-log[oh and ph+poh= 14 o o o the ph of the solution is 20.0. the concentration of oh ions is 1.0 x 10-8 m- the concentration of oh ions is 1.0 x 106 m- the ph of the solution is 8.0.
Answers: 3
You know the right answer?
When an ion‑selective electrode for x+ was immersed in 0.0482 m xcl, the measured potential was 0.04...
Questions


History, 23.09.2019 09:00


Mathematics, 23.09.2019 09:00


Mathematics, 23.09.2019 09:00





Mathematics, 23.09.2019 09:00

History, 23.09.2019 09:00

Mathematics, 23.09.2019 09:00


Health, 23.09.2019 09:00

Mathematics, 23.09.2019 09:00

Mathematics, 23.09.2019 09:00


Computers and Technology, 23.09.2019 09:00

Mathematics, 23.09.2019 09:00

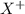 is 0.0861 M
is 0.0861 M![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{1}{[X]}](/tpl/images/0370/3205/84cb9.png)
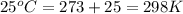
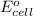 = standard electrode potential of the cell = ?
= standard electrode potential of the cell = ?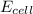 = emf of the cell = 0.0460 V
= emf of the cell = 0.0460 V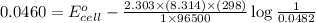
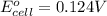
![0.0610=0.124-\frac{2.303\times (8.314)\times (298)}{1\times 96500}\log \frac{1}{[X]}](/tpl/images/0370/3205/50159.png)
![[X]=0.0861M](/tpl/images/0370/3205/21b33.png)


