
250 milliliters of a concentrated iron (ii) ion solution are sitting on the lab bench in need of analysis to determine its exact concentration of fe2+ ions. as part of the analysis a 15 ml sample of this solution was diluted to a final volume of 100 ml. the iron (ii) ion concentration of the diluted solution was found to be 1.35 x 10-3 mg/ml fe2+. what was the total number of milligrams of iron (ii) that were contained in the original solution?
note that this question is not asking for how many mg/ml of iron (ii) where in the original solution, but the total mass of iron contained in the original solution. hint: you must first calculate the mg/ml of iron (ii) in the original solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1


Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1
You know the right answer?
250 milliliters of a concentrated iron (ii) ion solution are sitting on the lab bench in need of ana...
Questions






Mathematics, 31.12.2019 19:31


Mathematics, 31.12.2019 19:31

Physics, 31.12.2019 19:31


Social Studies, 31.12.2019 19:31


Mathematics, 31.12.2019 19:31

Mathematics, 31.12.2019 19:31

Mathematics, 31.12.2019 19:31

Chemistry, 31.12.2019 19:31




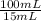 = 9,00x10⁻³ mg/mL
= 9,00x10⁻³ mg/mL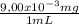 = 2,25 mg of Fe²⁺
= 2,25 mg of Fe²⁺


