
Chemistry, 12.11.2019 04:31 CameronVand21
Hardness in groundwater is due to the presence of metal ions, primarily mg2+ and ca2+ . hardness is generally reported as ppm caco3 . to measure water hardness, a sample of groundwater is titrated with edta, a chelating agent, in the presence of the indicator eriochrome black t, symbolized as in. eriochrome black t, a weaker chelating agent than edta, is red in the presence of ca2+ and turns blue when ca2+ is removed. red blue ca(in)2+ + edta ⟶ ca(edta)2+ + in a 50.00 ml sample of groundwater is titrated with 0.0450 m edta . if 13.70 ml of edta is required to titrate the 50.00 ml sample, what is the hardness of the groundwater in molarity and in parts per million of caco3 by mass? assume that ca2+ accounts for all of the hardness in the groundwater.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Hardness in groundwater is due to the presence of metal ions, primarily mg2+ and ca2+ . hardness is...
Questions

Mathematics, 24.05.2021 07:20

Mathematics, 24.05.2021 07:20



Chemistry, 24.05.2021 07:20

Social Studies, 24.05.2021 07:20

Mathematics, 24.05.2021 07:20


Mathematics, 24.05.2021 07:20








English, 24.05.2021 07:20



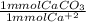 * 100.09 mg/mmol = 61.70 mg CaCO₃
* 100.09 mg/mmol = 61.70 mg CaCO₃

