
Chemistry, 12.11.2019 02:31 rubincain203
Write a balanced equation for the combustion of c7h16(l) (heptane) -- i. e. its reaction with o2(g) forming the products co2(g) and h2o(l). given the following standard heats of formation: δhf° of co2(g) is -393.5 kj/mol δhf° of h2o(l) is -286 kj/mol δhf° of c7h16(l) is -187.8 kj/mol what is the standard heat of reaction (δh°) for the combustion reaction of c7h16(l)? -4854.7 kj calculate the difference, δh-δe=δ(pv) for the combustion reaction of 1 mole of heptane. (assume standard state conditions and 298 k for all reactants and products.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
Write a balanced equation for the combustion of c7h16(l) (heptane) -- i. e. its reaction with o2(g)...
Questions


Chemistry, 04.05.2020 23:30







Mathematics, 04.05.2020 23:30




English, 04.05.2020 23:30



Mathematics, 04.05.2020 23:30




Mathematics, 04.05.2020 23:31

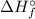 ) for: C₇H₁₆ (l) = -187.8 kJ/mol, O₂ (g) = 0 kJ/mol, CO₂ (g) = -393.5 kJ/mol, H₂O (l) = -286 kJ/mol
) for: C₇H₁₆ (l) = -187.8 kJ/mol, O₂ (g) = 0 kJ/mol, CO₂ (g) = -393.5 kJ/mol, H₂O (l) = -286 kJ/mol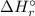 ) can be calculated by the Hess's law:
) can be calculated by the Hess's law:![\Delta H _{r}^{\circ } = \left [\sum \nu \cdot\Delta H _{f}^{\circ }(products) \right ] - \left [\sum \nu\cdot\Delta H _{f}^{\circ }(reactants) \right ]](/tpl/images/0369/9572/a156f.png)
 is the stoichiometric coefficient
is the stoichiometric coefficient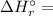
![\left [ 7\times \Delta H _{f}^{\circ }\left (CO_{2}\right )+ 8\times \Delta H _{f}^{\circ }\left (H_{2}O \right )\right ]](/tpl/images/0369/9572/3f95a.png)
![- \left [1\times \Delta H _{f}^{\circ }\left (C_{7}H_{16}\right ) +11\times \Delta H _{f}^{\circ }\left (O_{2} \right ) \right ]](/tpl/images/0369/9572/f3670.png)
![=\left [ 7\times \left (-393.5 kJ/mol \right )+ 8\times \left (-286 kJ/mol \right )\right ]](/tpl/images/0369/9572/7a112.png)
![-\left [1\times \left (-187.8 kJ/mol \right ) +11\times \left (0 kJ/mol \right ) \right ]](/tpl/images/0369/9572/be2b2.png)
![\Delta H _{r}^{\circ } = \left [ \left (-2754.5 \right )+ \left (-2288 \right )\right ]\left -[ \left (-187.8 \right ) +\left (0 \right )\right ]](/tpl/images/0369/9572/e3fd5.png)
![\Delta H _{r}^{\circ } = \left [ -5042.5 ]\left -[ -187.8] = \left ( -4854.7kJ \right )](/tpl/images/0369/9572/c52a2.png)


