Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1


Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
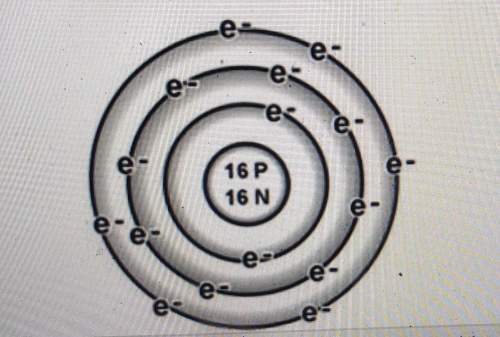
Is the atom below more likely to gain electrons or to lose electrons? explain how you can tell.
Questions

Mathematics, 16.03.2020 03:47


Mathematics, 16.03.2020 03:47

Mathematics, 16.03.2020 03:47

Mathematics, 16.03.2020 03:47




Mathematics, 16.03.2020 03:48

History, 16.03.2020 03:48


Mathematics, 16.03.2020 03:48



Mathematics, 16.03.2020 03:48

Mathematics, 16.03.2020 03:48

Mathematics, 16.03.2020 03:48

Mathematics, 16.03.2020 03:48

Mathematics, 16.03.2020 03:48