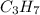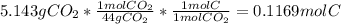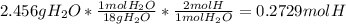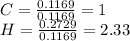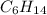A1.678 g sample of a component of the light petroleum distillate called naphtha is found to yield 5.143 g co2 (g) and 2.456 g h2o (l) on complete combustion. this particular compound is also found to be an alkane with one methyl group attached to a longer carbon chain and to have a molecular formula twice its empirical formula. the compound also has the following properties: melting point of -154 c , boiling point of 60.3 c , density of 0.6532 g/ml at 20 c , specific heat of 2.25 j/(g*c), and -204.6 kj/mol use the masses of carbon dioxide, co2, and water, h2o , to determine the empirical formula of the alkane component. express your answer as a chemical formula?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2


Chemistry, 23.06.2019 10:30
If a computer chip switches off -on-off in 0.015 us, what is the switching time in nanoseconds?
Answers: 2
You know the right answer?
A1.678 g sample of a component of the light petroleum distillate called naphtha is found to yield 5....
Questions


Chemistry, 10.03.2021 02:30

English, 10.03.2021 02:30



Mathematics, 10.03.2021 02:30

History, 10.03.2021 02:30

Mathematics, 10.03.2021 02:30

Mathematics, 10.03.2021 02:30

Mathematics, 10.03.2021 02:30


Mathematics, 10.03.2021 02:30

Mathematics, 10.03.2021 02:30

Computers and Technology, 10.03.2021 02:30

English, 10.03.2021 02:30

Mathematics, 10.03.2021 02:30

History, 10.03.2021 02:30

Mathematics, 10.03.2021 02:30


Mathematics, 10.03.2021 02:30