
Chemistry, 10.11.2019 06:31 electrofy456
Calculate δh o rxn for the following: a. sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh o f [sio2 (s)] = −910.9 kj/mol δh o f [hf (g)] = −273 kj/mol δh o f [sif4 (g)] = −1,614.9 kj/mol δh o f [h2o (l)] = −285.840 kj/mol b. c2h6(g) + o2(g) co2(g) + h2o(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
An occluded front moves over the farmland that has been experiencing drought conditions. what change in weather will this front likely bring? a. gray skies, but no rain b. an extended period of rain c. more dry air and sunny skies d. violent, short-lived thunderstorms
Answers: 3

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

You know the right answer?
Calculate δh o rxn for the following: a. sio2(s) + 4 hf(g) → sif4(g) + 2 h2o(l) δh o f [sio2 (s)] =...
Questions


English, 18.09.2019 21:00

Mathematics, 18.09.2019 21:00


History, 18.09.2019 21:00






Biology, 18.09.2019 21:00

Mathematics, 18.09.2019 21:00


History, 18.09.2019 21:00






Mathematics, 18.09.2019 21:00

 for the reaction is -183.68 kJ/mole
for the reaction is -183.68 kJ/mole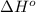
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0367/7523/45485.png)

![\Delta H^o_{rxn}=[(n_{(SiF_4)}\times \Delta H^o_f_{(SiF_4)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})]-[(n_{(SiO_2)}\times \Delta H^o_f_{(SiO_2)})+(n_{(HF)}\times \Delta H^o_f_{(HF)})]](/tpl/images/0367/7523/cf6d4.png)

![\Delta H^o_{rxn}=[(1\times -1614.9)+(2\times -285.840)]-[(1\times -910.9)+(4\times -273)]=-183.68kJ/mol](/tpl/images/0367/7523/c5c6f.png)


