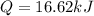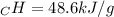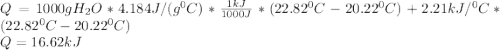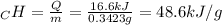Chemistry, 10.11.2019 06:31 angelica9613
A0.3423 g sample of pentane, c5h12, was burned in a bomb calorimeter. the temperature of the calorimeter and the 1.000kg of water contained therein rose from 20.22 degrees celcius to 22.82 degrees celcius. the heat capacity of the calorimeter is 2.21 kj/c. the heat capacity of water = 4.184 j/g c. a. how much heat was given off during combustion fo the sample of pentane. answer = 16.6 kjb. what is the heat of combustion, in kilojoules, per gram of pentaneanswer = 48.6 kj/g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

You know the right answer?
A0.3423 g sample of pentane, c5h12, was burned in a bomb calorimeter. the temperature of the calorim...
Questions




Health, 25.07.2019 12:50


Mathematics, 25.07.2019 12:50





History, 25.07.2019 12:50



Chemistry, 25.07.2019 12:50

English, 25.07.2019 12:50

Mathematics, 25.07.2019 12:50

Physics, 25.07.2019 12:50