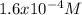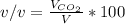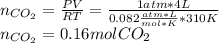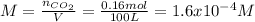Chemistry, 10.11.2019 06:31 missjohnson4449
Breathing air that contains 4.0% by volume co2 over time causes rapid breathing, throbbing headache, and nausea, among other symptoms. you may want to reference (pages 539 - 541) section 13.4 while completing this problem. part a what is the concentration of co2 in such air in terms of molarity, assuming 1 atm pressure and a body temperature of 37 ∘c? express you

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 13:30
The process by which liquid water changes into water vapour is called what ?
Answers: 2

Chemistry, 23.06.2019 14:30
If 125 cal of heat is applied to a 60.0-g piece of copper at 25.0 ? c , what will the final temperature be? the specific heat of copper is 0.0920 cal/(g? ? c) .
Answers: 1
You know the right answer?
Breathing air that contains 4.0% by volume co2 over time causes rapid breathing, throbbing headache,...
Questions

Mathematics, 30.01.2020 01:54



Mathematics, 30.01.2020 01:54


Mathematics, 30.01.2020 01:54

Mathematics, 30.01.2020 01:54

Mathematics, 30.01.2020 01:54



English, 30.01.2020 01:54


Chemistry, 30.01.2020 01:54

Computers and Technology, 30.01.2020 01:54

Mathematics, 30.01.2020 01:55

Biology, 30.01.2020 01:55


Mathematics, 30.01.2020 01:55

Mathematics, 30.01.2020 01:55

Mathematics, 30.01.2020 01:55