Chemistry, 10.11.2019 06:31 Alienchild239
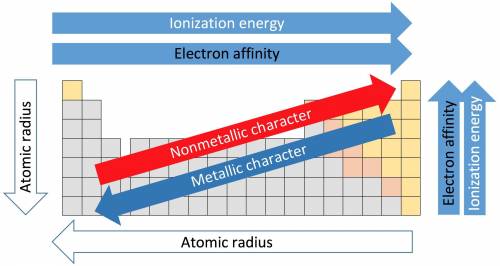
How do the periodic trends in metallic character compare to those for ionization energy? how do the periodic trends in metallic character compare to those for ionization energy? metals tend to have higher ionization energies than nonmetals. metals tend to have lower ionization energies than nonmetals. metals and nonmetals tend to have the same ionization energies.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
You know the right answer?
How do the periodic trends in metallic character compare to those for ionization energy? how do the...
Questions

Chemistry, 15.12.2019 08:31


Mathematics, 15.12.2019 08:31

History, 15.12.2019 08:31

Mathematics, 15.12.2019 08:31


Chemistry, 15.12.2019 08:31




Mathematics, 15.12.2019 08:31

Biology, 15.12.2019 08:31


Chemistry, 15.12.2019 08:31


Mathematics, 15.12.2019 08:31

Mathematics, 15.12.2019 08:31



History, 15.12.2019 08:31