
Chemistry, 10.11.2019 05:31 jalenclarke25
The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2co3) and the bicarbonate (hydrogen carbonate, hco3-) ion. what is the ratio of [bicarbonate]/[carbonic acid] at this ph? for carbonic acid, ka1 = 4.2 10-7.a) [bicarbonae]/[carbonic acid] = 0.11.b) [bicarbonate]/[carbonic acid] = 9.4.c) [bicarbonate]/[carbonic acid] = 0.38.d) none of the above ratios is correct. e) [bicarbonate]/[carbonic acid] = 2.65.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2...
Questions

History, 04.03.2020 19:19

Health, 04.03.2020 19:19



English, 04.03.2020 19:20




Mathematics, 04.03.2020 19:21

Mathematics, 04.03.2020 19:21



English, 04.03.2020 19:21



Mathematics, 04.03.2020 19:22

Computers and Technology, 04.03.2020 19:22



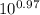 = [bicarbonate] / [carbonic acid] [bicarbonate] / [carbonic acid] = 9.33 ≈ 9.4
= [bicarbonate] / [carbonic acid] [bicarbonate] / [carbonic acid] = 9.33 ≈ 9.4

