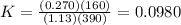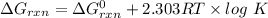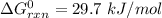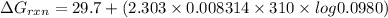Chemistry, 10.11.2019 05:31 elijahjwhite15
Consider the malate dehydrogenase reaction from the citric acid cycle. given the following concentrations, calculate the free energy change for this reaction at 37.0 °c (310 k). δg°\' for the reaction is 29.7 kj/mol. assume that the reaction occurs at ph 7. [malate] = 1.13 mm [oxaloacetate] = 0.270 mm [nad ] = 390 mm [nadh] = 160 mm

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 06:50
Organisms are classified as producer or consumers according to the way they ?
Answers: 1

Chemistry, 23.06.2019 07:30
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2

Chemistry, 23.06.2019 10:10
Which orbitals form a pi bond? a. the s orbital and three p orbitals b. the s orbital and two p orbitals c. overlapping p orbitals d. overlapping hybrid orbitals
Answers: 2

Chemistry, 23.06.2019 11:30
If 4.8 moles of x and 3.4 moles of y react according to the reaction below, how many moles of the excess reactant will be left over at the end of the reaction? 3x + 2y “yields”/ x3y2. a. 1.7 mol y left over b. 1.6 mol x left over c. 0.2 mol y left over d. 0.1 mol x left over
Answers: 1
You know the right answer?
Consider the malate dehydrogenase reaction from the citric acid cycle. given the following concentra...
Questions





Mathematics, 04.02.2020 23:54


Chemistry, 04.02.2020 23:54

Mathematics, 04.02.2020 23:54

Mathematics, 04.02.2020 23:54

Biology, 04.02.2020 23:54

History, 04.02.2020 23:54



Social Studies, 04.02.2020 23:54






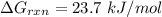
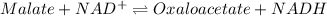
![K =\frac {[oxaloacetate][NADH]}{[malate][NAD^+]}](/tpl/images/0367/7081/e135a.png)
![[NAD^+]](/tpl/images/0367/7081/f5e9f.png) = 390 mM
= 390 mM