
The overall kf for the complex ion ag(nh3)2+ is 1.7 x 107. the ksp for agi is 8.5 x 10-17. what is the molar solubility of agi in a solution that is 2.0 m in nh3? the overall kf for the complex ion ag(nh3)2+ is 1.7 x 107. the ksp for agi is 8.5 x 10-17. what is the molar solubility of agi in a solution that is 2.0 m in nh3? 1.3 x 10-3 8.4 x 10-5 5.8 x 10-12 1.5 x 10-9 7.6 x 10-5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
The overall kf for the complex ion ag(nh3)2+ is 1.7 x 107. the ksp for agi is 8.5 x 10-17. what is t...
Questions




Mathematics, 15.07.2019 03:10

Mathematics, 15.07.2019 03:10




Mathematics, 15.07.2019 03:10



History, 15.07.2019 03:10

History, 15.07.2019 03:10



Mathematics, 15.07.2019 03:10

Mathematics, 15.07.2019 03:10



Mathematics, 15.07.2019 03:10

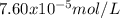
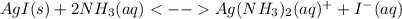
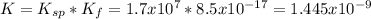
![K=\frac{[Ag(NH_3)_2^+][I^-]}{[NH_3]^2} \\K=\frac{x^2}{(2.0-2x)^2}\\\sqrt{K}= \sqrt{\frac{x^2}{(2.0-2x)^2}}\\2.0\sqrt{K}-2\sqrt{K}x-x=0\\7.60x10^{-5}-7.60x10^{-5}x-x=0\\x=\frac{7.60x10^{-5}}{1} =7.60x10^{-5}mol/L](/tpl/images/0367/6284/ce7cd.png)


