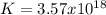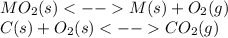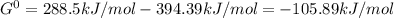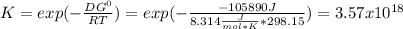Chemistry, 10.11.2019 04:31 shadowsnake
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s) < > m(s)+o2(g)delta g = 291.0 kj/mol1.) when the reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. what is the chemical equation of this coupled process? show that the reaction is in equilibrium, include physical states, and represent graphite as c(s) .) what is the thermodynamic equilibrium constant for the coupled reaction? k =

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2


Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1
You know the right answer?
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s...
Questions

Physics, 17.04.2020 21:33

Mathematics, 17.04.2020 21:33




Biology, 17.04.2020 21:33



Advanced Placement (AP), 17.04.2020 21:33

Mathematics, 17.04.2020 21:33



World Languages, 17.04.2020 21:33


Chemistry, 17.04.2020 21:33

History, 17.04.2020 21:33


Computers and Technology, 17.04.2020 21:33

History, 17.04.2020 21:33

English, 17.04.2020 21:33