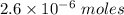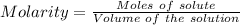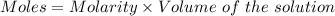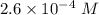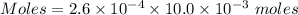Chemistry, 10.11.2019 03:31 shaylakabler333
Watch the video that shows using the dilution method to make a solution of known concentration. note that while the video shows the use of a beaker, in the lab we typically use a volumetric flask to make the diluted solution. the stock solution of kmno4 has a concentration of 2.6 × 10-4 m. the pipette has a volume of 10.0 ml. what is the amount of kmno4 delivered to the solution, in moles

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2


Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2
You know the right answer?
Watch the video that shows using the dilution method to make a solution of known concentration. note...
Questions

Chemistry, 30.01.2020 16:00


English, 30.01.2020 16:00

Chemistry, 30.01.2020 16:00


English, 30.01.2020 16:00


Social Studies, 30.01.2020 16:00

History, 30.01.2020 16:00

History, 30.01.2020 16:00








Health, 30.01.2020 16:00