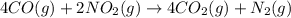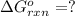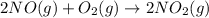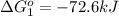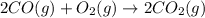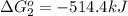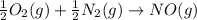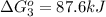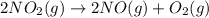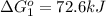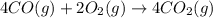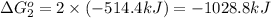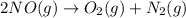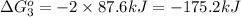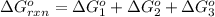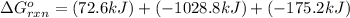Chemistry, 10.11.2019 01:31 TheOriginal2x
Calculate δg∘rxn for the following reaction: 4co(g)+2no2(g)→4co2(g)+n2(g).use the following reactions and given δg∘rxn values: a) 2no(g)+o2(g)→2no2(g), δg∘rxn= - 72.6 kjb) 2co(g)+o2(g)→2co2(g), δg∘rxn= - 514.4 kjc) 12o2(g)+12n2(g)→no(g), δg∘rxn= 87.6 kj

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2
You know the right answer?
Calculate δg∘rxn for the following reaction: 4co(g)+2no2(g)→4co2(g)+n2(g).use the following reaction...
Questions

Computers and Technology, 14.06.2021 17:40






Mathematics, 14.06.2021 17:40

Mathematics, 14.06.2021 17:40

Biology, 14.06.2021 17:40


Mathematics, 14.06.2021 17:40


Health, 14.06.2021 17:40

History, 14.06.2021 17:40


Geography, 14.06.2021 17:40


Social Studies, 14.06.2021 17:40

Mathematics, 14.06.2021 17:40

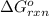 for the reaction is -1131.4 kJ
for the reaction is -1131.4 kJ