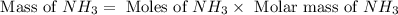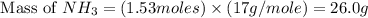Chemistry, 10.11.2019 01:31 maddiehope6140
Consider the following reaction: 2 no(g) + 5 h2(g) → 2 nh3(g) + 2 h2o(g) which set of solution maps would be needed to calculate the maximum amount of ammonia (nh3), in grams, that can be synthesized from 45.8 g of nitrogen monoxide (no) and 12.4 g of hydrogen (h2)? i. g no → mol no → mol nh3 → g nh3 ii. g h2 → mol h2 → mol nh3 → g nh3 iii. g no → mol no → mol h2o → g h2o iv. g h2 → mol h2 → mol h2o → g h2o

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Acar tire has a pressure of 2.38 atm at 15.2 c. if the pressure inside reached 4.08 atm, the tire will explode. how hot would the tire have to get for this to happen? report the temperature in degrees celsius.
Answers: 2

Chemistry, 21.06.2019 14:30
Which statement justifies that phosphine (ph3) is a polar molecule?
Answers: 1

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3
You know the right answer?
Consider the following reaction: 2 no(g) + 5 h2(g) → 2 nh3(g) + 2 h2o(g) which set of solution maps...
Questions

Advanced Placement (AP), 09.06.2021 06:20

Mathematics, 09.06.2021 06:20



Mathematics, 09.06.2021 06:30

Biology, 09.06.2021 06:30



Mathematics, 09.06.2021 06:30

Mathematics, 09.06.2021 06:30







Mathematics, 09.06.2021 06:30

Social Studies, 09.06.2021 06:30

Mathematics, 09.06.2021 06:30

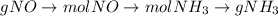
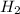 = 12.4 g
= 12.4 g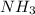 = 17 g/mole
= 17 g/mole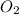 .
.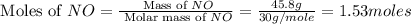
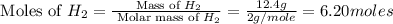
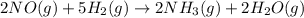
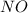 react with 5 mole of
react with 5 mole of 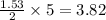 moles of
moles of