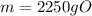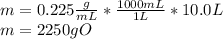Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which of the following mining methods disrupts the sea floor?
Answers: 1

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
Acertain organic compound o has a solubility in hexena of 0.225 at 10. °c. calculate the greatest ma...
Questions

History, 16.03.2020 23:28



Mathematics, 16.03.2020 23:28








Computers and Technology, 16.03.2020 23:28







Health, 16.03.2020 23:28

Mathematics, 16.03.2020 23:28