
Chemistry, 09.11.2019 05:31 elijahlylejamez45
Ammonia is produced commercially by the direct reaction of the elements. the formation of 1.00 moles of gaseous nh3 by this reaction releases 46.17 kj of heat. how much energy (in kj) is released, when 28.0 kg of hydrogen gas, h2, reacts in excess nitrogen gas, n2?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 23.06.2019 09:00
Spaghetti sauce can be high in sodium. what is a good guideline for mg of sodium per half cup serving? a. less than 1 mg b. less than 800 mg c. less than 700 mg d. less than 400 mg
Answers: 2

Chemistry, 23.06.2019 18:30
Why must pencil be used to draw on paper in paper chromatography
Answers: 2
You know the right answer?
Ammonia is produced commercially by the direct reaction of the elements. the formation of 1.00 moles...
Questions

Physics, 22.07.2019 00:00

Biology, 22.07.2019 00:00



Arts, 22.07.2019 00:00

Chemistry, 22.07.2019 00:00

Mathematics, 22.07.2019 00:00

History, 22.07.2019 00:00


History, 22.07.2019 00:00


Mathematics, 22.07.2019 00:00

English, 22.07.2019 00:00




Chemistry, 22.07.2019 00:00



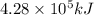 of heat energy will be released
of heat energy will be released
 moles of
moles of  produces 1 mol of
produces 1 mol of 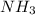 to release 46.17 kJ of heat.
to release 46.17 kJ of heat.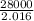 moles of
moles of 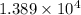 moles of
moles of 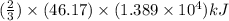 heat or
heat or 

