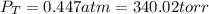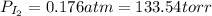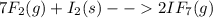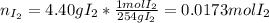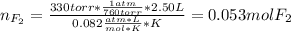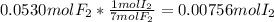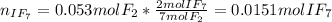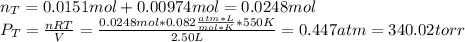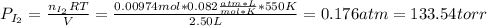Chemistry, 09.11.2019 05:31 jimennacastillo15
When gaseous f2 and solid i2 are heated to high temperatures, the i2 sublimes and gaseous iodine heptafluoride forms.3.30 × 102 torr of f2 and 4.40 g of solid i2 are put into a 2.50 l container at 2.50 × 102 k and the container is heated to 5.50 × 10^2 k.
(a) what is the final pressure?
ptotal =
(b) what is the partial pressure of i2 gas?
pi2 = i2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
When gaseous f2 and solid i2 are heated to high temperatures, the i2 sublimes and gaseous iodine hep...
Questions


Mathematics, 15.07.2021 22:00



World Languages, 15.07.2021 22:00

Mathematics, 15.07.2021 22:00

Mathematics, 15.07.2021 22:00

English, 15.07.2021 22:00





Mathematics, 15.07.2021 22:00

Geography, 15.07.2021 22:00


Mathematics, 15.07.2021 22:00

Mathematics, 15.07.2021 22:00


History, 15.07.2021 22:00

Mathematics, 15.07.2021 22:00