
Chemistry, 09.11.2019 00:31 haydencheramie
Imagine that you are in chemistry lab and need to make 1.00 l of a solution with a ph of 2.80. you have in front of you 100 ml of 7.00×10−2 m hcl, 100 ml of 5.00×10−2 m naoh, and plenty of distilled water. you start to add hcl to a beaker of water when someone asks you a question. when you return to your dilution, you accidentally grab the wrong cylinder and add some naoh. once you realize your error, you assess the situation. you have 84.0 ml of hcl and 88.0 ml of naoh left in their original containers. part a assuming the final solution will be diluted to 1.00 l , how much more hcl should you add to achieve the desired ph?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3
You know the right answer?
Imagine that you are in chemistry lab and need to make 1.00 l of a solution with a ph of 2.80. you h...
Questions

Mathematics, 18.02.2021 01:10

Social Studies, 18.02.2021 01:10


Mathematics, 18.02.2021 01:10

History, 18.02.2021 01:10

Chemistry, 18.02.2021 01:10



Social Studies, 18.02.2021 01:10


Chemistry, 18.02.2021 01:10


Mathematics, 18.02.2021 01:10

Computers and Technology, 18.02.2021 01:10






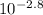 = [H⁺][H⁺] = 1.58 x 10⁻³ MThen we use the definition of Molarity [C=n/V]:[H⁺] = 1.58 x 10⁻³ M = molesH⁺ / 1.00 LmolesH⁺ = 1.58 x 10⁻³
= [H⁺][H⁺] = 1.58 x 10⁻³ MThen we use the definition of Molarity [C=n/V]:[H⁺] = 1.58 x 10⁻³ M = molesH⁺ / 1.00 LmolesH⁺ = 1.58 x 10⁻³


