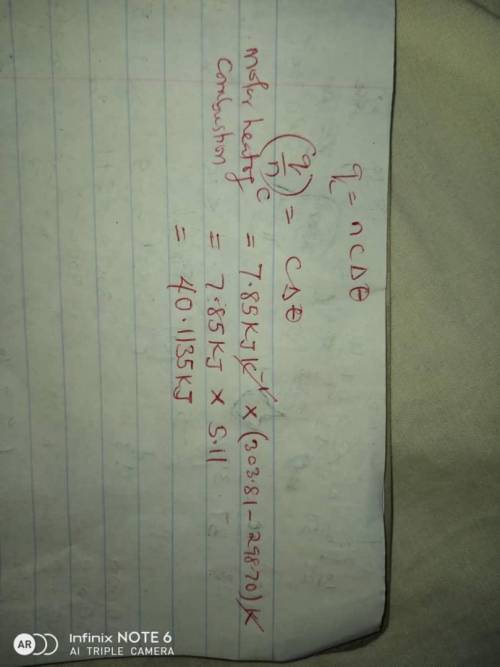Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
A1.11 g sample of caffeine, c8h10n4o2, burns in a constant-volume calorimeter that has a heat capaci...
Questions








History, 23.07.2019 12:30

Mathematics, 23.07.2019 12:30


History, 23.07.2019 12:30

Mathematics, 23.07.2019 12:30


Computers and Technology, 23.07.2019 12:30