Chemistry, 08.11.2019 23:31 astultz309459
Suppose 200.0 ml of 1.00 m hcl and 200.0 ml of 1.00 m naoh, both initially at 21.0°c, are mixed in a thermos flask. when the reaction is complete, the temperature is 27.8°c. assuming that the solutions have the same heat capacity as pure water, compute the heat released (in kj).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
You know the right answer?
Suppose 200.0 ml of 1.00 m hcl and 200.0 ml of 1.00 m naoh, both initially at 21.0°c, are mixed in a...
Questions



English, 09.03.2022 21:40

Physics, 09.03.2022 21:40


Mathematics, 09.03.2022 21:40


Mathematics, 09.03.2022 21:40








Mathematics, 09.03.2022 21:50

History, 09.03.2022 21:50

Mathematics, 09.03.2022 21:50

Mathematics, 09.03.2022 21:50


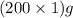 = 200 g
= 200 g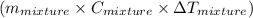
 represents change in temperature
represents change in temperature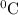 )
)![[400g\times 4.186J.g^{-1}.^{0}\textrm{C}^{-1}\times(27.8-21.0)^{0}\textrm{C} ]](/tpl/images/0366/2490/9c7ad.png) =
= 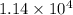 J=11.4 kJ
J=11.4 kJ