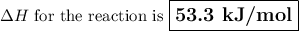Chemistry, 08.11.2019 22:31 debrielcalderon
Use hess's law to calculate the enthalpy change for the reaction: 3c(s) + 3h2(g) → c3h6(g) given the following thermochemical equations: 2c3h6(g) + 9o2(g) → 6co2(g) + 6h2o(l) δh = -4182.6 kj/mol c(s) + o2(g) → co2(g) δh = -393.51 kj/mol h2(g) + ½o2(g) → h2o(l) δh = -285.83 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
Use hess's law to calculate the enthalpy change for the reaction: 3c(s) + 3h2(g) → c3h6(g) given th...
Questions






Computers and Technology, 29.07.2020 22:01





Mathematics, 29.07.2020 22:01



Mathematics, 29.07.2020 22:01

Mathematics, 29.07.2020 22:01


Biology, 29.07.2020 22:01