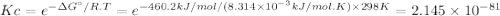Chemistry, 08.11.2019 21:31 brookicooki99
The δg°f of atomic oxygen is 230.1 kj/mol. find δg° for the following dissociation reactiono2 (g) < --> 2o (g)then calculate its equilibrium constant at 298 k.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2
You know the right answer?
The δg°f of atomic oxygen is 230.1 kj/mol. find δg° for the following dissociation reactiono2 (g) &l...
Questions




Social Studies, 11.02.2022 02:40

Chemistry, 11.02.2022 02:40






English, 11.02.2022 02:40

Mathematics, 11.02.2022 02:40

Mathematics, 11.02.2022 02:40

Business, 11.02.2022 02:40


Mathematics, 11.02.2022 02:40

Computers and Technology, 11.02.2022 02:40

Computers and Technology, 11.02.2022 02:40

Chemistry, 11.02.2022 02:40

Mathematics, 11.02.2022 02:40