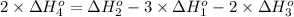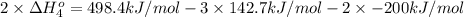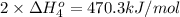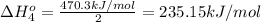Chemistry, 08.11.2019 07:31 destinystanley3794
Calculate the standard reaction enthalpy for the reaction no2(g) → no(g) + o(g) given +142.7 kj/mol for the standard enthalpy of formation of ozone and o2(g) → 2 o(g) ∆h ◦ = +498.4 kj/mol no(g) + o3(g) → no2(g) + o2(g) ∆h◦ = −200 kj/mol remember the definition of the standard enthalpy of formation of a substance.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
Calculate the standard reaction enthalpy for the reaction no2(g) → no(g) + o(g) given +142.7 kj/mol...
Questions


Biology, 18.10.2020 16:01


History, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

History, 18.10.2020 16:01




English, 18.10.2020 16:01


Mathematics, 18.10.2020 16:01

History, 18.10.2020 16:01


English, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

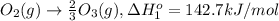 ..[1]
..[1]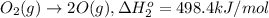 ..[2]
..[2]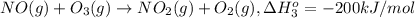 ..[3]
..[3]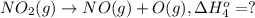 ..[4]
..[4]