
Chemistry, 08.11.2019 06:31 exoticbunnylover123
7.23 th e condensation reaction of acetone, (ch3)2co (propanone), in aqueous solution is catalyzed by bases, b, which react reversibly with acetone to form the carbanion c3h5o−. th e carbanion then reacts with a molecule of acetone to give the product. a simplifi ed version of the mechanism is (1) ah + b → bh+ + a− (2) a− + bh+ → ah + b (3) a− + ha → product where ah stands for acetone and a− its carbanion. use the steadystate approximation to fi nd the concentration of the carbanion and derive the rate equation for the formation of the product.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1
You know the right answer?
7.23 th e condensation reaction of acetone, (ch3)2co (propanone), in aqueous solution is catalyzed b...
Questions

Mathematics, 16.10.2019 17:50

Social Studies, 16.10.2019 17:50


Computers and Technology, 16.10.2019 17:50


Biology, 16.10.2019 17:50





Chemistry, 16.10.2019 17:50


History, 16.10.2019 18:00




English, 16.10.2019 18:00



![\frac{d[product]}{dt}=K_{3}[A^{-}][HA]](/tpl/images/0365/1783/52b0c.png)
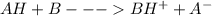

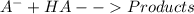
![\frac{d[A^{-}]}{dt}=K_{1}[AH][B] - K_{2}[A^{-}][BH^{+}]-K_{3}[A^{-}][HA]](/tpl/images/0365/1783/3cbda.png) ...(4)
...(4)![\frac{d[B]}{dt}=-K_{1}[AH][B] +K_{2}[A^{-}][BH^{+}]=0](/tpl/images/0365/1783/4d945.png)
![[B]=\frac{K_{2}[A^{-}][BH^{+}]}{K_{1}[AH]}](/tpl/images/0365/1783/bbdf9.png) ....(5)
....(5)![\frac{d[A^{-}]}{dt}=K_{1}[AH][\frac{K_{2}[A^{-}][BH^{+}]}{K_{1}[AH]} - K_{2}[A^{-}][BH^{+}]-K_{3}[A^{-}][HA]](/tpl/images/0365/1783/08b5f.png)
![\frac{d[A^{-}]}{dt}=-K_{3}[A^{-}][HA]](/tpl/images/0365/1783/bd67b.png)


