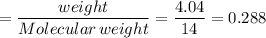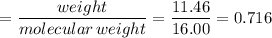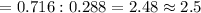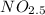Chemistry, 08.11.2019 02:31 kkeith121p6ujlt
If 4.04g of n combine with 11.46g o to produce a compound with a molar mass of 108.0 g/mol, what is the molecular formula of this compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2
You know the right answer?
If 4.04g of n combine with 11.46g o to produce a compound with a molar mass of 108.0 g/mol, what is...
Questions

Biology, 29.12.2019 23:31


Mathematics, 29.12.2019 23:31

Mathematics, 29.12.2019 23:31



Mathematics, 29.12.2019 23:31

Mathematics, 29.12.2019 23:31

History, 29.12.2019 23:31

Mathematics, 29.12.2019 23:31



Mathematics, 29.12.2019 23:31



Chemistry, 29.12.2019 23:31

Mathematics, 29.12.2019 23:31


Computers and Technology, 29.12.2019 23:31