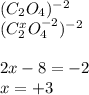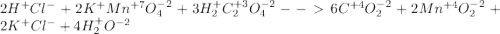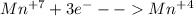Chemistry, 08.11.2019 01:31 JamesLachoneus
In an ion, the sum of the oxidation states is equal to the overall ionic charge. note that the sign of the oxidation states and the number of atoms associated with each oxidation state must be considered. in oh-, for example, the oxygen atom has an oxidation state of 2 and the hydrogen atom has an oxidation state of +1, for a total of ( -2 ) + ( +1 ) = -1
(i) what is the oxidation state of each individual carbon atom in c2o4 2^-
express the oxidation state numerically (e. g., +1).
(ii) which element is reduced in this reaction?
2hcl+ 2kmno4 + 3h2c2o4 ? 6co2 +2mno2 + 2kcl + 4h2o

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

You know the right answer?
In an ion, the sum of the oxidation states is equal to the overall ionic charge. note that the sign...
Questions

Mathematics, 13.03.2021 14:00

English, 13.03.2021 14:00



Mathematics, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00



Mathematics, 13.03.2021 14:00



History, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00


History, 13.03.2021 14:00


Mathematics, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00