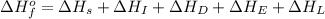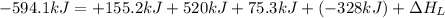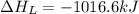Chemistry, 07.11.2019 22:31 kraigstlistt
The process of forming an ionic salt from its constituent metallic and nonmetallic elements is called the born-haber cycle, which is a series of thermochemical processes, each with a δh, that add up (think of hess’s law) to complete a 5 step process for the formation of the salt. given the following data, calculate the lattice energy per mole of lif(s) formed. li(s) → li(g) δh°

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2


Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
The process of forming an ionic salt from its constituent metallic and nonmetallic elements is calle...
Questions




English, 17.09.2019 15:50

Mathematics, 17.09.2019 15:50



Health, 17.09.2019 15:50


Mathematics, 17.09.2019 15:50


English, 17.09.2019 15:50

Mathematics, 17.09.2019 15:50


Mathematics, 17.09.2019 15:50




Chemistry, 17.09.2019 15:50

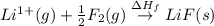
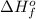 = enthalpy of formation of lithium fluoride = -594.1 kJ
= enthalpy of formation of lithium fluoride = -594.1 kJ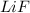 :
: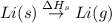
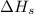 = sublimation energy of lithium = +155.2 kJ
= sublimation energy of lithium = +155.2 kJ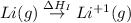
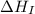 = ionization energy of lithium = +520 kJ
= ionization energy of lithium = +520 kJ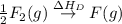
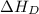 = dissociation energy of fluorine = +75.3 kJ
= dissociation energy of fluorine = +75.3 kJ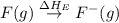
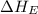 = electron affinity energy of fluorine = -328 kJ
= electron affinity energy of fluorine = -328 kJ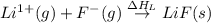
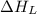 = lattice energy of lithium fluoride = ?
= lattice energy of lithium fluoride = ?