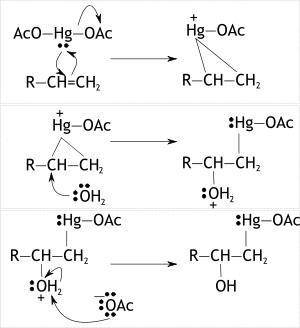
Alkenes can be converted to alcohols by reaction with mercuric acetate to form a ? -hydroxyalkylmercury(ii) acetate compound, a reaction called oxymercuration. subsequent reduction with nabh4 reduces the c? hg bond to a c? h bond, forming the alkyl alcohol, a reaction called demercuration. draw the structures of the hg-containing compound(s) and the final alcohol product(s) formed in the following reaction sequence, omitting, by products. if applicable, draw hydrogen at a chirality center and indicate stereochemistry via wedge-and-dash bonds.
neutral produst (s) of oxymercuration. include stereoisomers, if any
image from custom entry tool hg(oocch3)2
>
h2o, thf

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

You know the right answer?
Alkenes can be converted to alcohols by reaction with mercuric acetate to form a ? -hydroxyalkylmerc...
Questions

















Mathematics, 08.10.2021 19:00



Mathematics, 08.10.2021 19:00