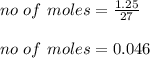Assuming you start with 1.25 g of pure aluminum, calculate the following:
1) the amount of po...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1


Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
Questions

Mathematics, 08.10.2021 23:50


English, 08.10.2021 23:50



Mathematics, 08.10.2021 23:50


Mathematics, 08.10.2021 23:50

Mathematics, 08.10.2021 23:50


Mathematics, 08.10.2021 23:50

English, 08.10.2021 23:50

Social Studies, 08.10.2021 23:50

Mathematics, 08.10.2021 23:50


Mathematics, 08.10.2021 23:50


Mathematics, 08.10.2021 23:50

Biology, 08.10.2021 23:50