
A0.05270.0527 g piece of metal containing an unknown amount of silver is dissolved to form 25.00 ml of solution. to the solution, 35.435.4 ml of 0.02190.0219 m kcl kcl is added, resulting in the complete precipitation of the silver ash agcl agcl . the excess cl−cl− added to the solution is then back titrated with a standard 0.02190.0219 m agno3 agno3 solution. a total of 14.3314.33 ml of the standard nano3 agno3 solution is required to reach the silver chromate end point in the mohr method. calculate the percent silver in the piece of metal.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
A0.05270.0527 g piece of metal containing an unknown amount of silver is dissolved to form 25.00 ml...
Questions


Social Studies, 20.11.2021 14:00

Mathematics, 20.11.2021 14:00

Mathematics, 20.11.2021 14:00

Social Studies, 20.11.2021 14:00



Mathematics, 20.11.2021 14:00


History, 20.11.2021 14:00


History, 20.11.2021 14:00


Social Studies, 20.11.2021 14:00

Physics, 20.11.2021 14:00





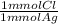 = 0.314 mmol Cl⁻
= 0.314 mmol Cl⁻

