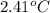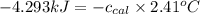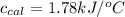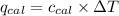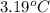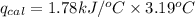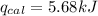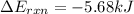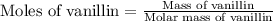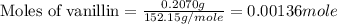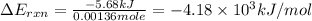The combustion of 0.1625 g benzoic acid increases the temperature of a bomb calorimeter by 2.41°c. calculate the heat capacity of this calorimeter. (the energy released by combustion of benzoic acid is 26.42 kj/g.)
kj/°c
a 0.2070 g sample of vanillin (c8h8o3) is then burned in the same calorimeter, and the temperature increases by 3.19°c. what is the energy of combustion per gram of vanillin?
kj/g
what is the energy of combustion per mole of vanillin?
kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
The combustion of 0.1625 g benzoic acid increases the temperature of a bomb calorimeter by 2.41°c. c...
Questions



History, 04.02.2020 09:04


History, 04.02.2020 09:04

Computers and Technology, 04.02.2020 09:04




Mathematics, 04.02.2020 09:04

Physics, 04.02.2020 09:04

Mathematics, 04.02.2020 09:04


Mathematics, 04.02.2020 09:04

Mathematics, 04.02.2020 09:04




Social Studies, 04.02.2020 09:04

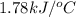
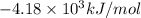
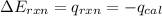
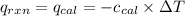
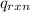 = heat released by the reaction = -4.293 kJ
= heat released by the reaction = -4.293 kJ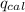 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter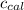 = specific heat of calorimeter = ?
= specific heat of calorimeter = ? = change in temperature =
= change in temperature =