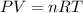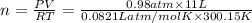Chemistry, 07.11.2019 05:31 mckleinrivero
Amaterials scientist has created an alloy containing aluminum, copper, and zinc, and wants to determine the percent composition of the alloy. the scientist takes a 13.039 g sample of the alloy and reacts it with concentrated hcl . the reaction converts all of the aluminum and zinc in the alloy to aluminum chloride and zinc chloride in addition to producing hydrogen gas. the copper does not react with the hcl . upon completion of the reaction, a total of 11 l of hydrogen gas was collected at a pressure of 744 torr and a temperature of 27.0 °c . additionally, 2.761 g of unreacted copper is recovered. calculate the mass of hydrogen gas formed from the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
Amaterials scientist has created an alloy containing aluminum, copper, and zinc, and wants to determ...
Questions




French, 17.12.2020 19:20

English, 17.12.2020 19:20

Advanced Placement (AP), 17.12.2020 19:20

Mathematics, 17.12.2020 19:20


Mathematics, 17.12.2020 19:30

Mathematics, 17.12.2020 19:30

English, 17.12.2020 19:30

Mathematics, 17.12.2020 19:30

Mathematics, 17.12.2020 19:30


History, 17.12.2020 19:30


Mathematics, 17.12.2020 19:30



Mathematics, 17.12.2020 19:30