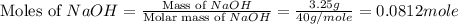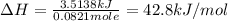Chemistry, 07.11.2019 05:31 heybrothwrlogan
When a 3.25 g sample of solid sodium hydroxide was dissolved in a calorimeter in 100.0 g of water, the temperature rose from 23.9 °c to 32.0 °c. calculate ∆h (in kj/mol) for the solution process: naoh (s) → na+ (aq) + oh- (aq)
use a calorimeter heat capacity of ccal = 15.8 j/°c

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
When a 3.25 g sample of solid sodium hydroxide was dissolved in a calorimeter in 100.0 g of water, t...
Questions

Biology, 19.09.2019 21:00

Mathematics, 19.09.2019 21:00




Advanced Placement (AP), 19.09.2019 21:00




Biology, 19.09.2019 21:00

Mathematics, 19.09.2019 21:00

Mathematics, 19.09.2019 21:00

Social Studies, 19.09.2019 21:00

History, 19.09.2019 21:00

Computers and Technology, 19.09.2019 21:00

Mathematics, 19.09.2019 21:00

Mathematics, 19.09.2019 21:00

Social Studies, 19.09.2019 21:00


![q=[q_1+q_2]](/tpl/images/0363/3965/341bc.png)
![q=[c_1\times \Delta T+m\times c_2\times \Delta T]](/tpl/images/0363/3965/21bf4.png)
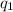 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter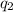 = heat absorbed by the water
= heat absorbed by the water = specific heat of calorimeter =
= specific heat of calorimeter = 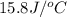
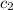 = specific heat of water =
= specific heat of water = 
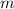 = mass of water = 100.0 g
= mass of water = 100.0 g = change in temperature =
= change in temperature = 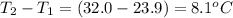
![q=[(15.8J/^oC\times 8.1^oC)+(100.0g\times 4.18J/g^oC\times 8.1^oC)]](/tpl/images/0363/3965/4042f.png)
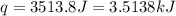 (1 kJ = 1000 J)
(1 kJ = 1000 J)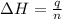
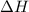 = enthalpy change = ?
= enthalpy change = ?