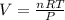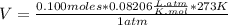Chemistry, 07.11.2019 04:31 genyjoannerubiera
Magnesium metal (0.100 mol) and hydrochloric acid (0.500 mol hcl) are combined and react to completion. what volume of hydrogen gas, measured at stp, is produced? mg(s) + 2hcl(aq) → mgcl2(aq) + h2(g) (r = 0.08206 l • atm/k • mol) select one: a. 22.4 l of h2 b. 5.60 l of h2 c. 4.48 l of h2 d. 11.2 l of h2 e. 2.24 l of h2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Magnesium metal (0.100 mol) and hydrochloric acid (0.500 mol hcl) are combined and react to completi...
Questions



Mathematics, 18.02.2021 21:10

Mathematics, 18.02.2021 21:10

Chemistry, 18.02.2021 21:10



Mathematics, 18.02.2021 21:10





History, 18.02.2021 21:10

Mathematics, 18.02.2021 21:10

Mathematics, 18.02.2021 21:10

Mathematics, 18.02.2021 21:10

Mathematics, 18.02.2021 21:10



Social Studies, 18.02.2021 21:10

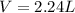 of
of 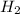
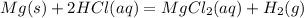
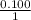
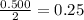
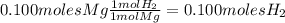
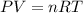 , where P is the pressure, at STP P=1 atm, V is the volume, n is the number of moles, R is a constante whose value is R=0.08206
, where P is the pressure, at STP P=1 atm, V is the volume, n is the number of moles, R is a constante whose value is R=0.08206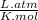 , and T is the temperature, at STP T=273K
, and T is the temperature, at STP T=273K