
During a spontaneous chemical reaction, it is found that δssys is less than 0. this means that group of answer choices δssurr is less than 0 and its magnitude is less than δssys. δssurr is less than 0 and its magnitude is greater than δssys. δssurr is greater than 0 and its magnitude is less than δssys. δssurr is greater than 0 and its magnitude is greater than δssys. an error has been made, as ssys is greater than 0 by necessity for a spontaneous process.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
During a spontaneous chemical reaction, it is found that δssys is less than 0. this means that group...
Questions

Mathematics, 26.07.2019 23:20

Health, 26.07.2019 23:20

Mathematics, 26.07.2019 23:20


Mathematics, 26.07.2019 23:20

Mathematics, 26.07.2019 23:20

English, 26.07.2019 23:20


Mathematics, 26.07.2019 23:20


Mathematics, 26.07.2019 23:20



Mathematics, 26.07.2019 23:20






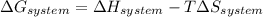
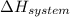 is the enthalpy change and
is the enthalpy change and 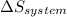 is the entropy change of the system.
is the entropy change of the system.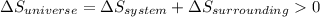
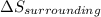 should be greater than 0 or positive. Also,
should be greater than 0 or positive. Also, 


