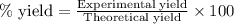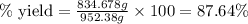Ether, (c2h5)2o, which was originally used as an anesthetic but has been replaced by safer and more effective medications, is prepared by the reaction of ethanol with sulfuric acid. what is the percent yield of ether if 1.17 l (d = 0.7134 g/ml) is isolated from the reaction of 1.500 l of c2h5oh (d = 0.7894 g/ml)? 2 c2h5oh + h2so4 → (c2h5)2o + h2so4 · h2o

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Ether, (c2h5)2o, which was originally used as an anesthetic but has been replaced by safer and more...
Questions

History, 24.04.2020 19:43


Mathematics, 24.04.2020 19:43


Computers and Technology, 24.04.2020 19:43






Biology, 24.04.2020 19:43


History, 24.04.2020 19:43


Mathematics, 24.04.2020 19:44

Mathematics, 24.04.2020 19:44

Law, 24.04.2020 19:44

Mathematics, 24.04.2020 19:44

English, 24.04.2020 19:44

Mathematics, 24.04.2020 19:44


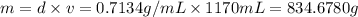
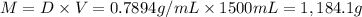
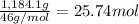
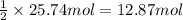 of an ether.
of an ether.