
Chemistry, 07.11.2019 01:31 kaitlynmeats
Consider the titration of 20.00 ml of 0.754 m sodium benzoate with a solution of 0.525 m nitric acid. a. calculate the equivalence volume in ml. b. calculate the ph at the equivalence point. c. calculate the ph of the solution after addition of 16.20 ml nitric acid. d. calculate the ph of the solution after addition of 39.82 ml nitric acid.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
Consider the titration of 20.00 ml of 0.754 m sodium benzoate with a solution of 0.525 m nitric acid...
Questions




English, 17.07.2019 09:00

Chemistry, 17.07.2019 09:00

English, 17.07.2019 09:00



Mathematics, 17.07.2019 09:00


History, 17.07.2019 09:00

Physics, 17.07.2019 09:00

Biology, 17.07.2019 09:00


History, 17.07.2019 09:00

Biology, 17.07.2019 09:00

Biology, 17.07.2019 09:00

English, 17.07.2019 09:00

Biology, 17.07.2019 09:00

History, 17.07.2019 09:00

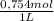 = 0,01508mol of NaBz
= 0,01508mol of NaBz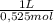 = 0,0287L = 28,7 mL
= 0,0287L = 28,7 mL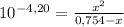
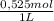 = 5,838x10⁻³moles of H⁺. The volume is 20,0mL + 39,82mL = 59,82mL ≡ 0,05982L. Thus, [H⁺] is:
= 5,838x10⁻³moles of H⁺. The volume is 20,0mL + 39,82mL = 59,82mL ≡ 0,05982L. Thus, [H⁺] is:

