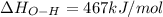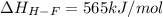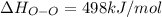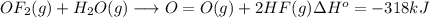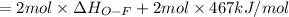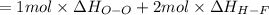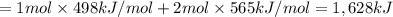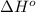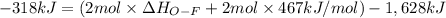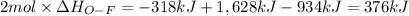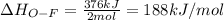Chemistry, 07.11.2019 01:31 jenlicavoli
Oxygen difluoride is an unstable molecule that reacts readily with water. calculate the bond energy of the o–f bond using the standard enthalpy of reaction and the bond energy data provided. just enter a number (no units). of2(g) + h2o(g) \longrightarrow⟶ o=o(g) + 2hf(g) \deltaδh° = –318 kj bond: o–h o=o h–f bond energy (kj/mol): 467 498 565

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
Oxygen difluoride is an unstable molecule that reacts readily with water. calculate the bond energy...
Questions



Mathematics, 21.09.2019 04:00






Chemistry, 21.09.2019 04:00



Health, 21.09.2019 04:00

Mathematics, 21.09.2019 04:00

English, 21.09.2019 04:00


History, 21.09.2019 04:00


Biology, 21.09.2019 04:00

Mathematics, 21.09.2019 04:00