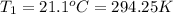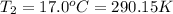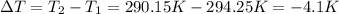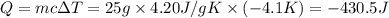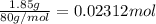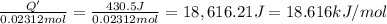Instant cold packs used to ice athletic injuries on the field contain ammonium nitrate and water separated by a thin plastic divider. when the divider is broken, the ammonium nitrate dissolves according to the endothermic reaction: nh4no3 (s) --> nh4+ (aq) + no3- (aq) 1.85g ammonium nitrate is added to water in a calorimeter. the total solution (water and ammonium nitrate) is 25.0g. the heat capacity of the calorimeter is ccal=45.0 j/k. the initial temperature of the solution is 21.1c. the final temperature of the solution is 17.0c. assume cs of the solution is 4.20 j/(g k) what is δhrxn per mol of the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
Instant cold packs used to ice athletic injuries on the field contain ammonium nitrate and water sep...
Questions



Mathematics, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31



Mathematics, 19.12.2019 07:31




Mathematics, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31

Biology, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31



History, 19.12.2019 07:31


Mathematics, 19.12.2019 07:31