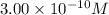Asolution contains 0.021 m cl and 0.017 m i. a solution containing copper (i) ions is added to selectively precipitate one of the ions. at what concentration of copper (i) ion will a precipitate begin to form? what is the identity of the precipitate? ksp(cucl) = 1.0 × 10-6, ksp(cui) = 5.1 × 10-12.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

You know the right answer?
Asolution contains 0.021 m cl and 0.017 m i. a solution containing copper (i) ions is added to selec...
Questions

Geography, 26.10.2020 18:30

Mathematics, 26.10.2020 18:30

Social Studies, 26.10.2020 18:30

Chemistry, 26.10.2020 18:30

History, 26.10.2020 18:30

History, 26.10.2020 18:30




Geography, 26.10.2020 18:30

Mathematics, 26.10.2020 18:30



Mathematics, 26.10.2020 18:30



Mathematics, 26.10.2020 18:30

Mathematics, 26.10.2020 18:30

Business, 26.10.2020 18:30

Mathematics, 26.10.2020 18:30

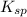 of CuCl =
of CuCl = 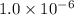
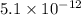
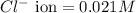
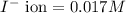
![K_{sp}=[Cu^+][Cl^-]](/tpl/images/0361/6888/73cc7.png)
![1.0\times 10^{-6}=[Cu^+]\times 0.021](/tpl/images/0361/6888/d87ff.png)
![[Cu^+]=\frac{1.0\times 10^{-6}}{0.021}=4.76\times 10^{-5}M](/tpl/images/0361/6888/3549a.png)
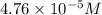
![K_{sp}=[Cu^+][I^-]](/tpl/images/0361/6888/38825.png)
![5.1\times 10^{-12}=[Cu^+]\times 0.017](/tpl/images/0361/6888/99563.png)
![[Cu^+]=\frac{5.1\times 10^{-12}}{0.017}=3.00\times 10^{-10}M](/tpl/images/0361/6888/762ae.png)