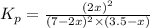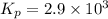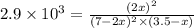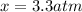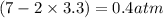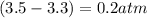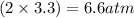Chemistry, 06.11.2019 04:31 PlsHelpMeh3401
Calculate the pressures of no, cl2, and nocl in an equilibrium mixture produced by the reaction of a starting mixture with 7.0 atm no and 3.5 atm cl2. (hint: kp is relatively large; assume the reaction goes to completion then comes back to equilibrium.)
2 no(g) + cl2(g) --> 2 nocl(g)kp = 2.9 ✕ 103 at 149°c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

You know the right answer?
Calculate the pressures of no, cl2, and nocl in an equilibrium mixture produced by the reaction of a...
Questions


Mathematics, 10.10.2020 22:01

Business, 10.10.2020 22:01




History, 10.10.2020 22:01




Mathematics, 10.10.2020 22:01

Mathematics, 10.10.2020 22:01


Mathematics, 10.10.2020 22:01

Mathematics, 10.10.2020 22:01

Mathematics, 10.10.2020 22:01




Mathematics, 10.10.2020 22:01

 ,
, 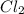 , and
, and 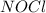 in an equilibrium mixture are 0.4 atm , 0.2 atm and 6.6 atm respectively.
in an equilibrium mixture are 0.4 atm , 0.2 atm and 6.6 atm respectively.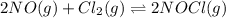
![K_p=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0361/4899/09f8c.png)